Satellite-like cells
Robust, reliable and reproducible production of early progenitor cells for disease modeling and regenerative medicine including both localized and systemic skeletal muscle diseases
Learn moreSatellite-like cells
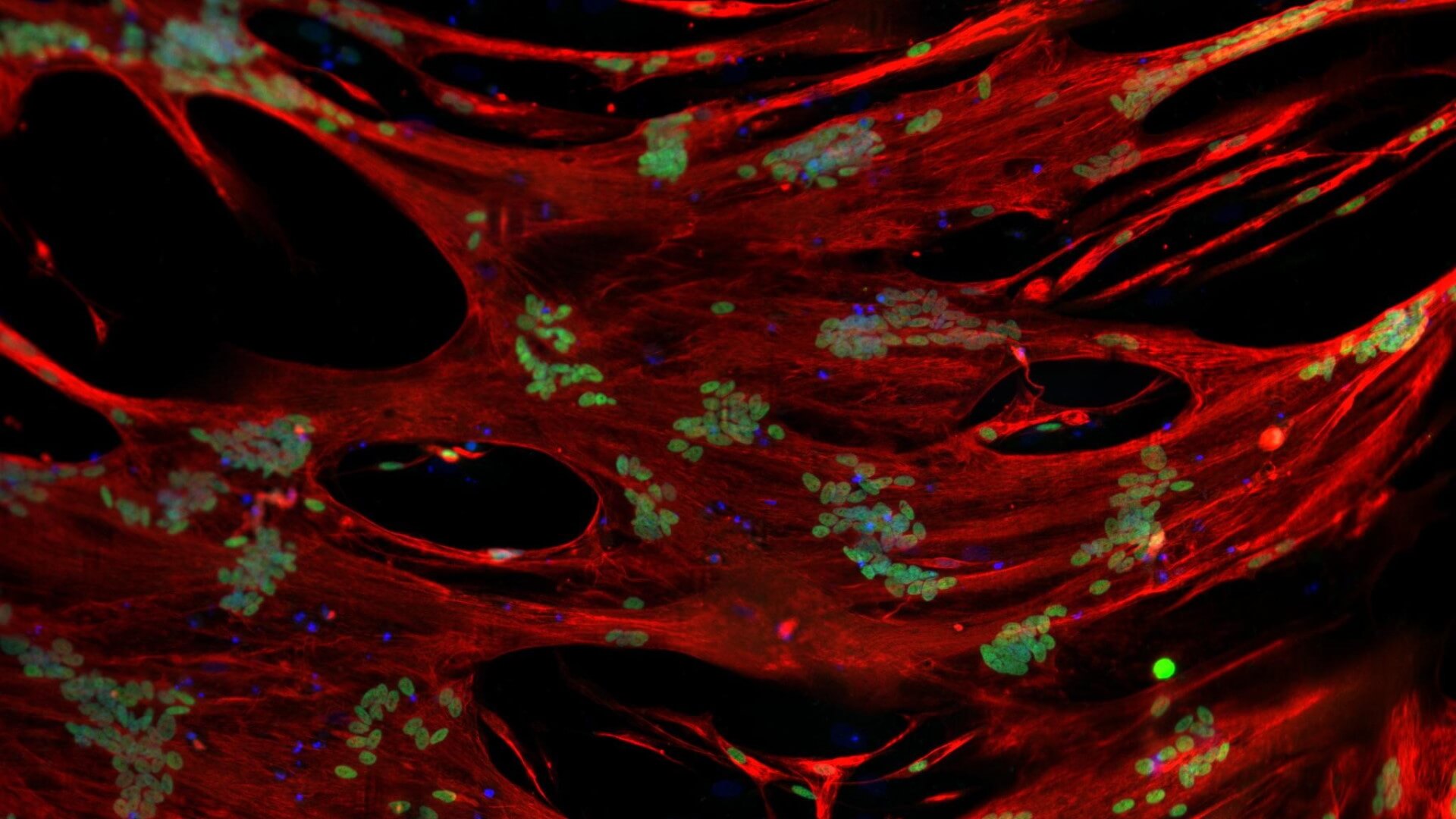
Skeletal muscle regeneration and maintenance involve well-orchestrated and precisely regulated modulation of various cellular and molecular responses. Satellite cells also known as skeletal muscle stem cells are fundamental to this process. The self-renewing proliferation of satellite cells regiments the stem cell population and provides diverse myogenic cells. These progenitors proliferate, differentiate, fuse and lead to new myofiber formation. Satellite cells also exhibit non-cycling behavior or quiescence. Our team has been working towards identification of functions of satellite cells and their niche during the process of skeletal muscle regeneration and maintenance in both healthy and disease states. Using a combination of finely tuned conditions and advanced specialized high content screening techniques, we have identified optimized conditions to generate a population of sells that show distinct molecular and cellular characteristics of satellite cells. We termed them ‘satellite-like cells’ or SLCs. We further validated their highly migratory and regenerative potential in vivo (Figs 1-3).
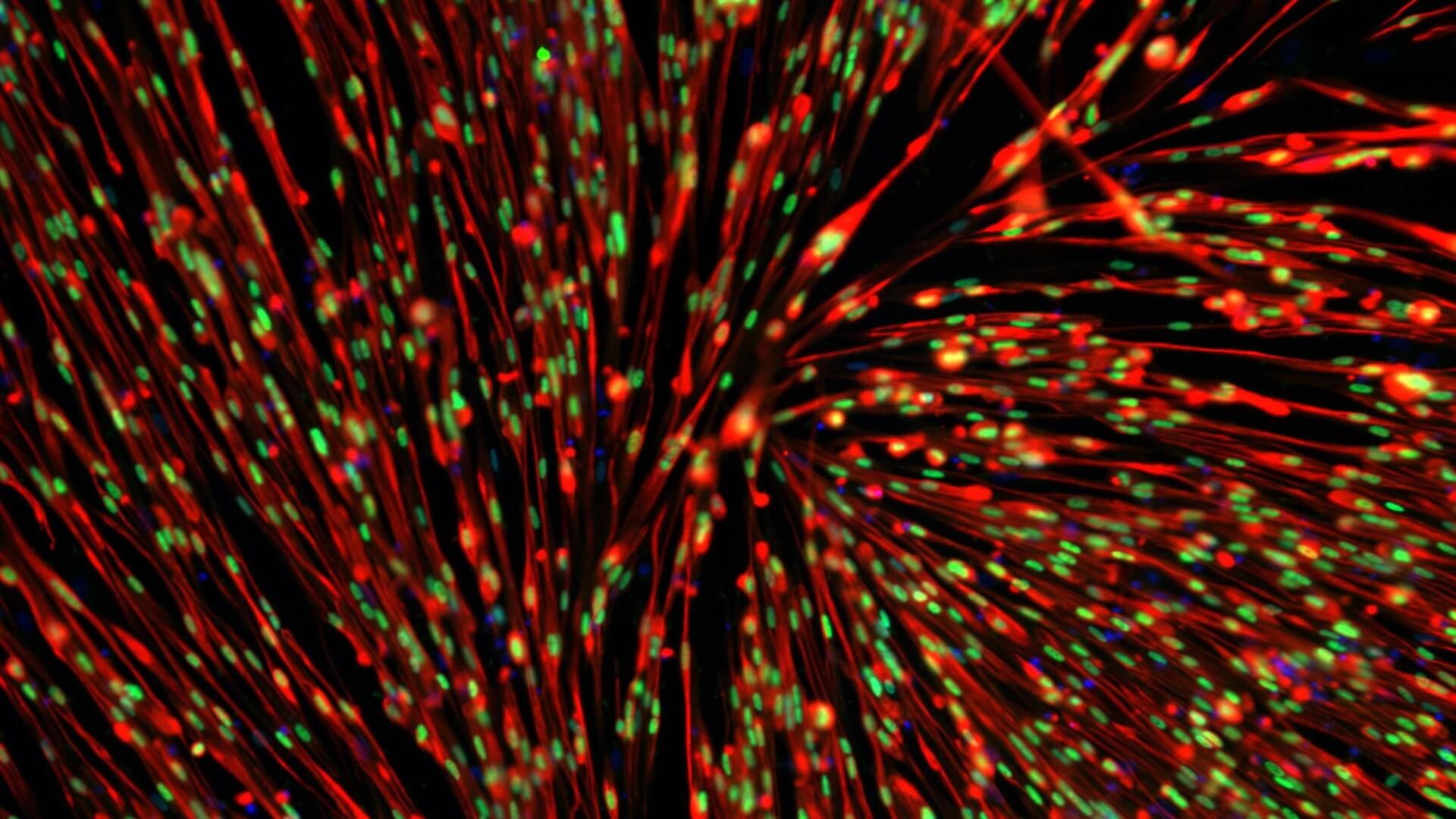
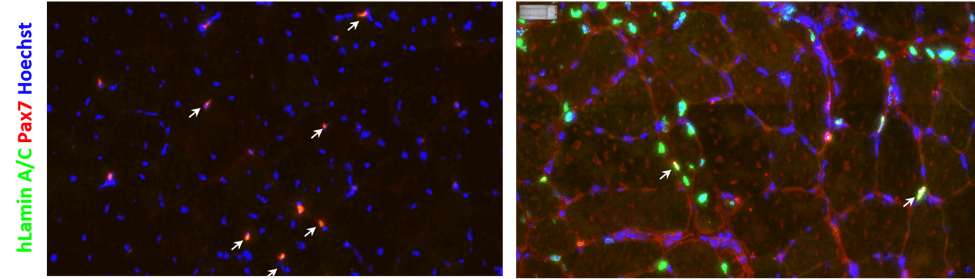 Fig1. Myocea’s SLC (Left): most interstitial human nuclei (hLaminA/C+ cells) are PAX7+; non-myogenic cells are not observed; human skeletal muscle myoblasts (HSMM, Right) : most cells are PAX7-, highly heterogeneous, contain many non-myogenic cells
Fig1. Myocea’s SLC (Left): most interstitial human nuclei (hLaminA/C+ cells) are PAX7+; non-myogenic cells are not observed; human skeletal muscle myoblasts (HSMM, Right) : most cells are PAX7-, highly heterogeneous, contain many non-myogenic cells
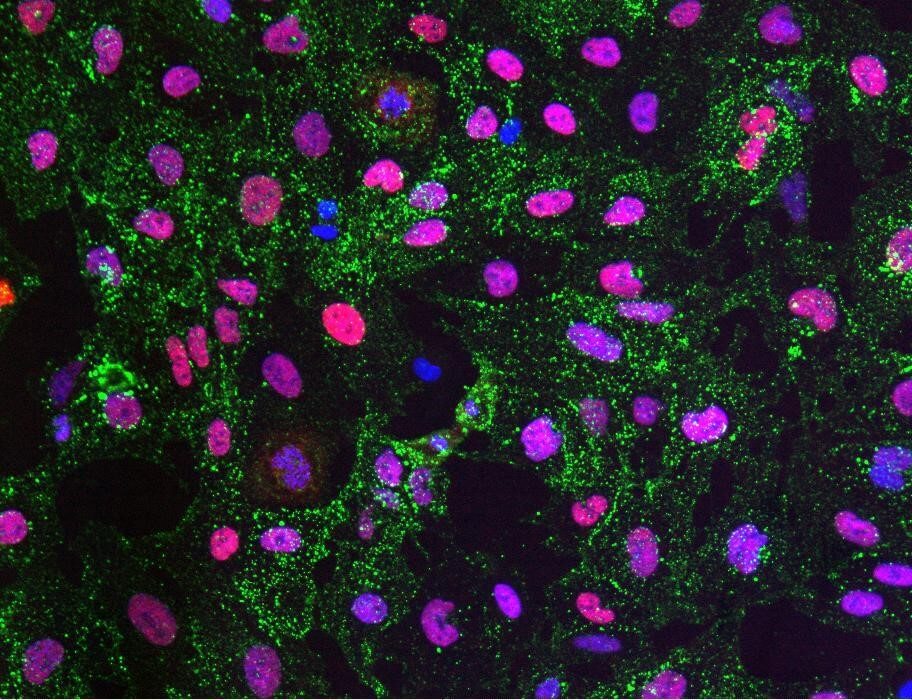
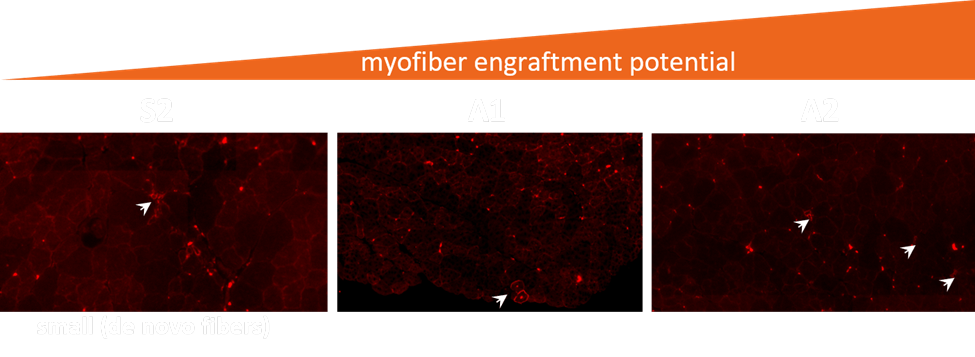 Fig2. Myocea’s SLCs contribute to myogenesis in vivo (murine xenograft model), several alternative conditions (S2, A1 and A2) are shown.
Fig2. Myocea’s SLCs contribute to myogenesis in vivo (murine xenograft model), several alternative conditions (S2, A1 and A2) are shown.
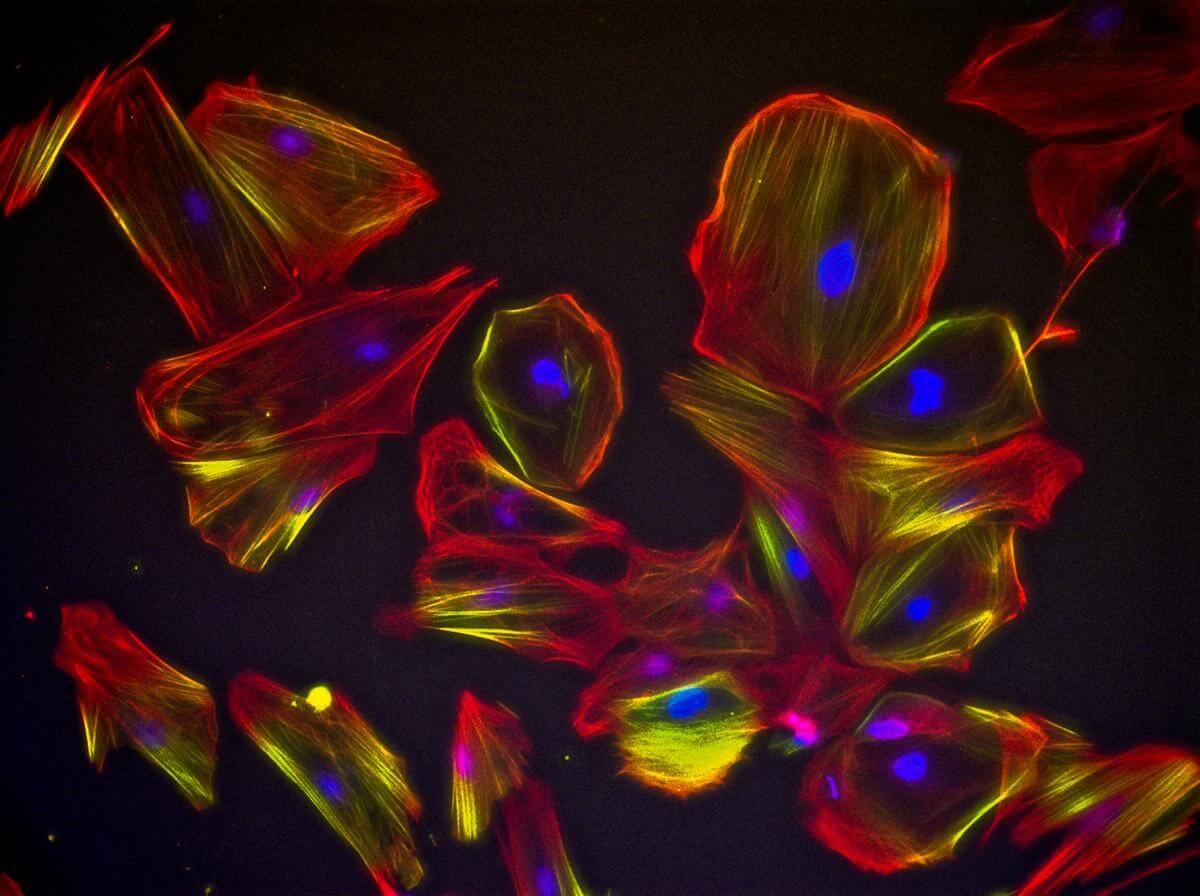
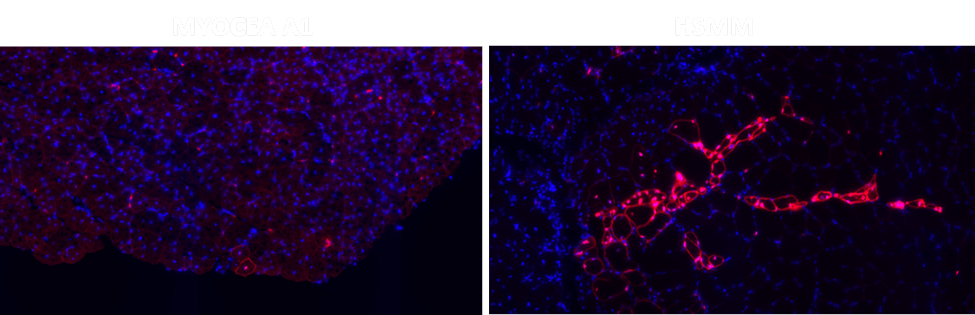 Fig3. Human skeletal muscle myoblasts (HSMM, Right) make clustered myofibers whereas Myocea’s SLCs are more dispersed and migrate to a broader area of muscle damage signifying better regenerative potential.
Fig3. Human skeletal muscle myoblasts (HSMM, Right) make clustered myofibers whereas Myocea’s SLCs are more dispersed and migrate to a broader area of muscle damage signifying better regenerative potential.
Distinctive features of our satellite-like cells are:
- all populations of Myocea’s SLC are capable of differentiating into myofibers
- all groups are highly migratory compared to human skeletal muscle myoblasts (HSMM)
- highly dynamic, migratory behavior compared to myoblasts that display low migratory capacities
- show no evidence of scarring and non-myogenic differentiation
- make small de novo fibers and engraft SATC niche in vivo
- PAX7+ staining and location adjacent to myofibers are maintained through 8 weeks following reinjury
- integral to disease modeling of muscular dystrophies and is used internally for disease-relevant screening to find small molecule or biologics-based therapies
- potential to develop unique xenograft disease models
- potential to develop a cell therapy platform to treat various muscle diseases and/or
- identify agents with therapeutic potential to modulate SATC niche in vivo
High yield
No sorting
No genetic manipulation
Skeletal Muscle Differentiation Media
How to order:

US and Europe:
AMSBio

Japan:
Funakoshi
Bulk and all other inquiries:

Myocea Inc.:
info@myocea.com
Contact Us
Want to say hello? Want to know more about us? Give us a call or drop us an email and we will get back to you as soon as we can.