Facioscapulohumeral Muscular Dystrophy (FSHD)
Facioscapulohumeral muscular dystrophy (FSHD) is a monogenetic muscle disorder in which the muscles of the face (facies), shoulder blades (scapula), and upper arms (humerus bone) are the most affected. FSHD patients exhibit progressive muscle weakness and atrophy. FSHD is the third most common type of muscular dystrophy, behind Duchenne and Becker muscular dystrophies (D/BMD) and myotonic dystrophy (DM1/2).
Learn moreMYOCEA: Focus on Muscular Diseases
Therapies, Modeling, Platforms: Differentiators
- Pipeline:
- Facioscapulohumeral dystrophy (FSHD): small molecule therapeutics GBC0905
- Orphan Drug Designation granted in May’18
- IND is on track to be filed in 1Q2021
- screening: SBMA (Kennedy's Disease), SMA (skeletal muscle component of the neuromuscular junction), treatment of the local muscle disease
- Skeletal Muscle Media
- Standalone commercial module
- Validated with > 50 external contacts (pharma, academia)
- Skeletal muscle progenitors
- Modeling, screening, therapeutics for regeneration
- Satellite-like cells, myoblasts, custom myotubes
- Diagnostics: markers of disease progression, efficacy
- Partnership with Pangenia: DNA/RNA, protein markers
- Longitudinal assessment of therapeutic effect, individual response
FSHD Cause, Pathophysiology
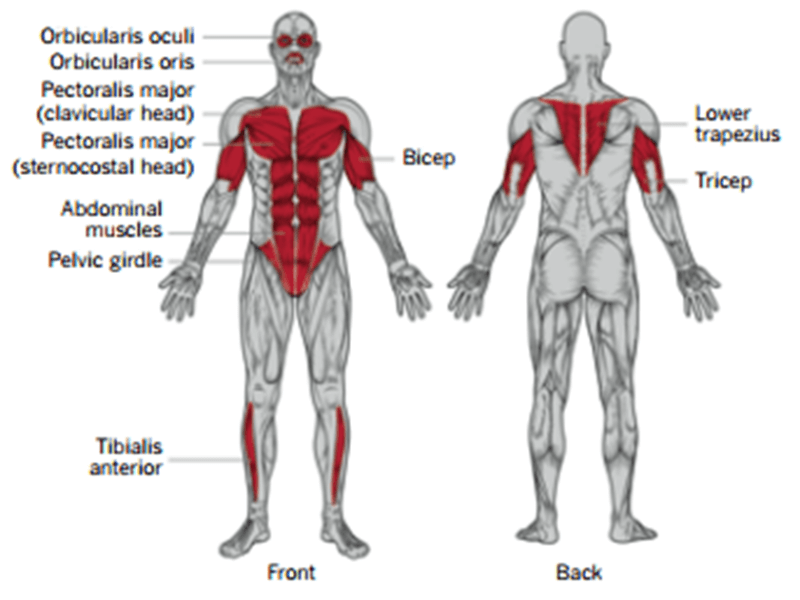
- Progressive, painful muscle wasting, affects most skeletal muscles
- Difficulty smiling, drinking through straw
- Inability to lift hands above shoulder height, winged scapula
- Foot drop (~¼ of patients are wheelchair dependent)
- Muscle to fat transition by MRI
- ‘Orphan’ disease
- 1 in 8,000 people affected (about 900,000 worldwide)
- Up to 40,000 people affected in the US
- No disease-specific treatment
- Multiple clinical, pre-clinical efforts
- Typical onset: late teens to early 30s
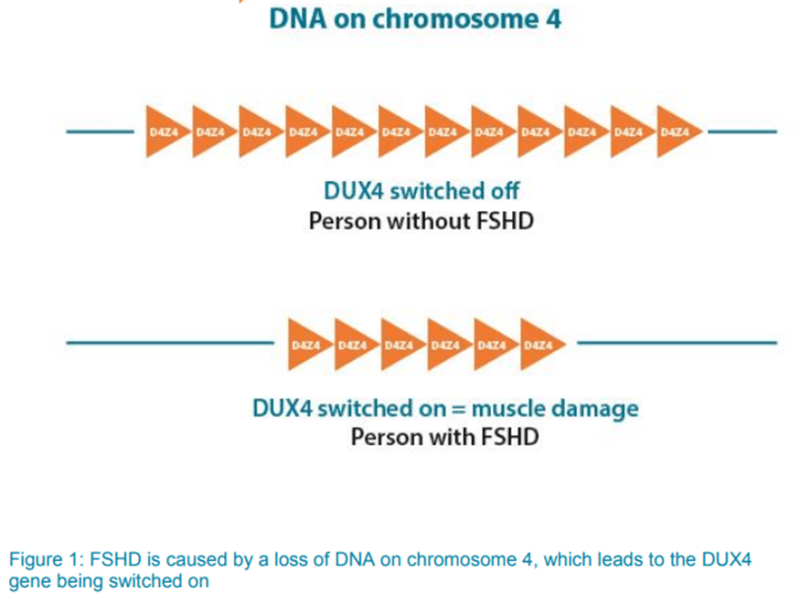
- Cause:
- expression of DUX4 protein in FSHD muscle
- few copies, but extremely toxic to cells
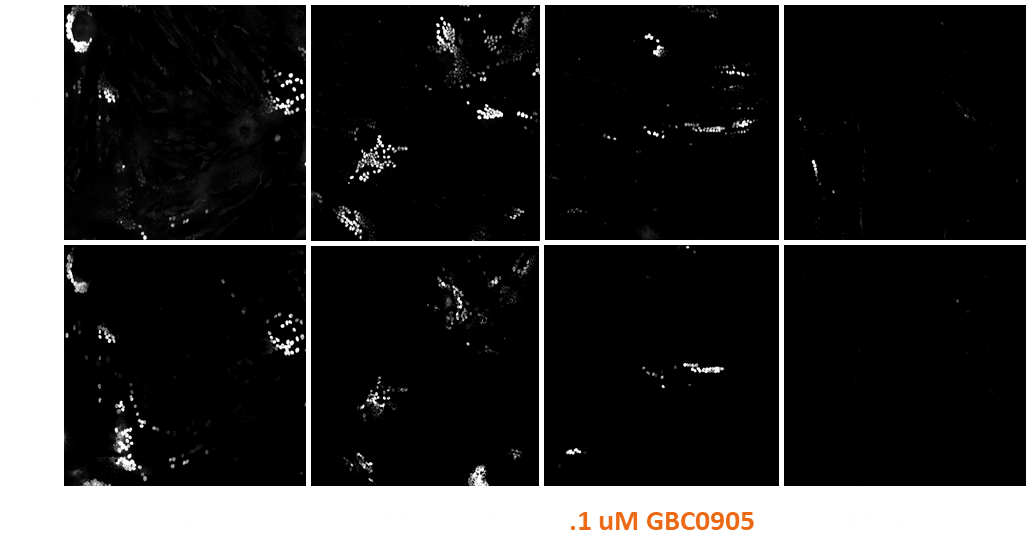
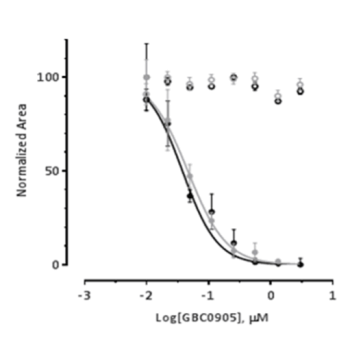
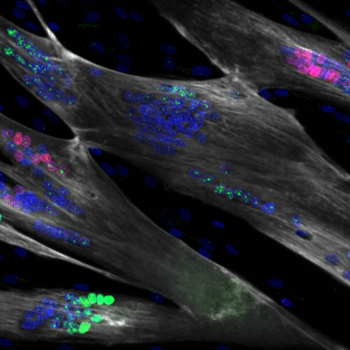
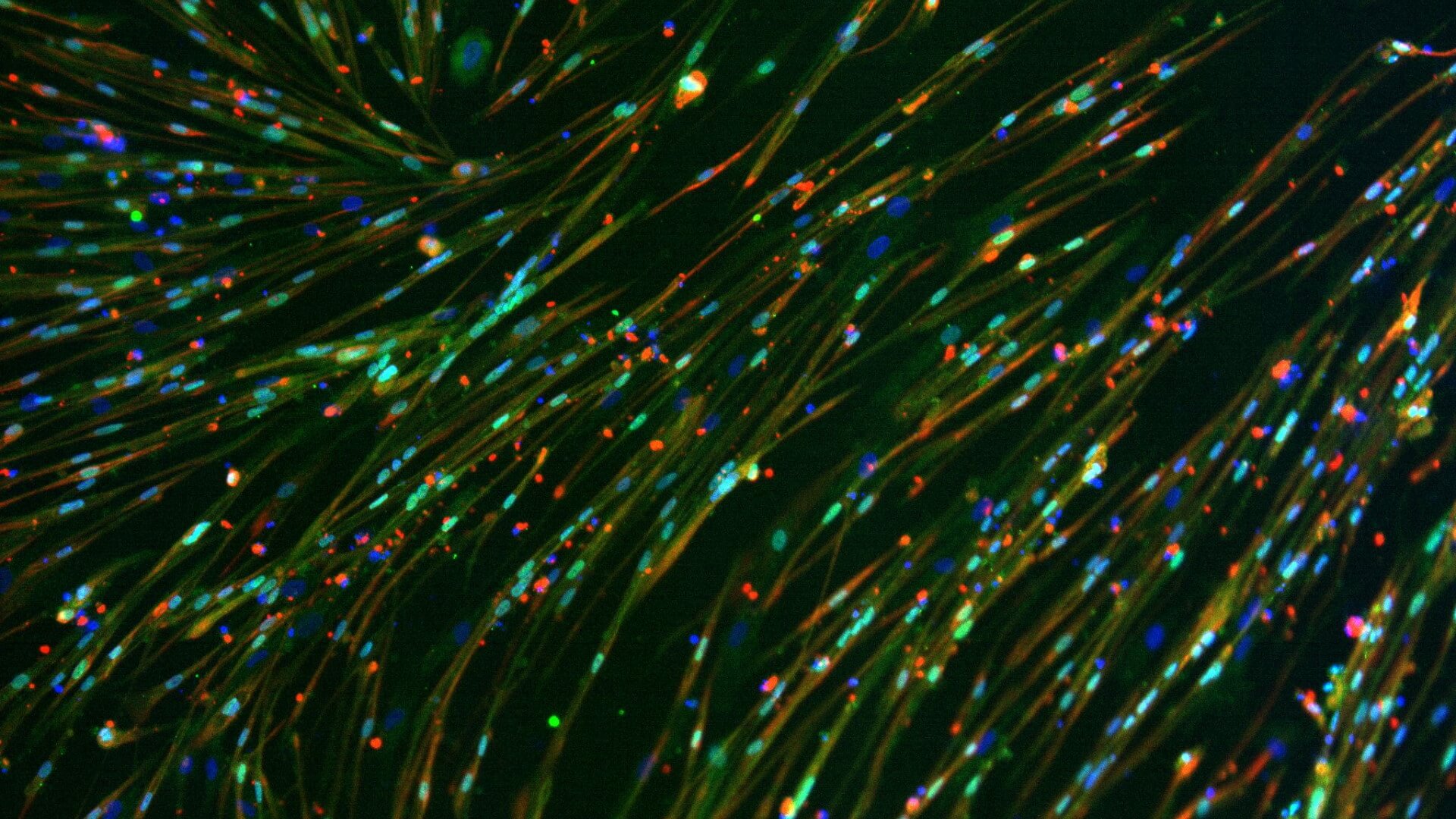
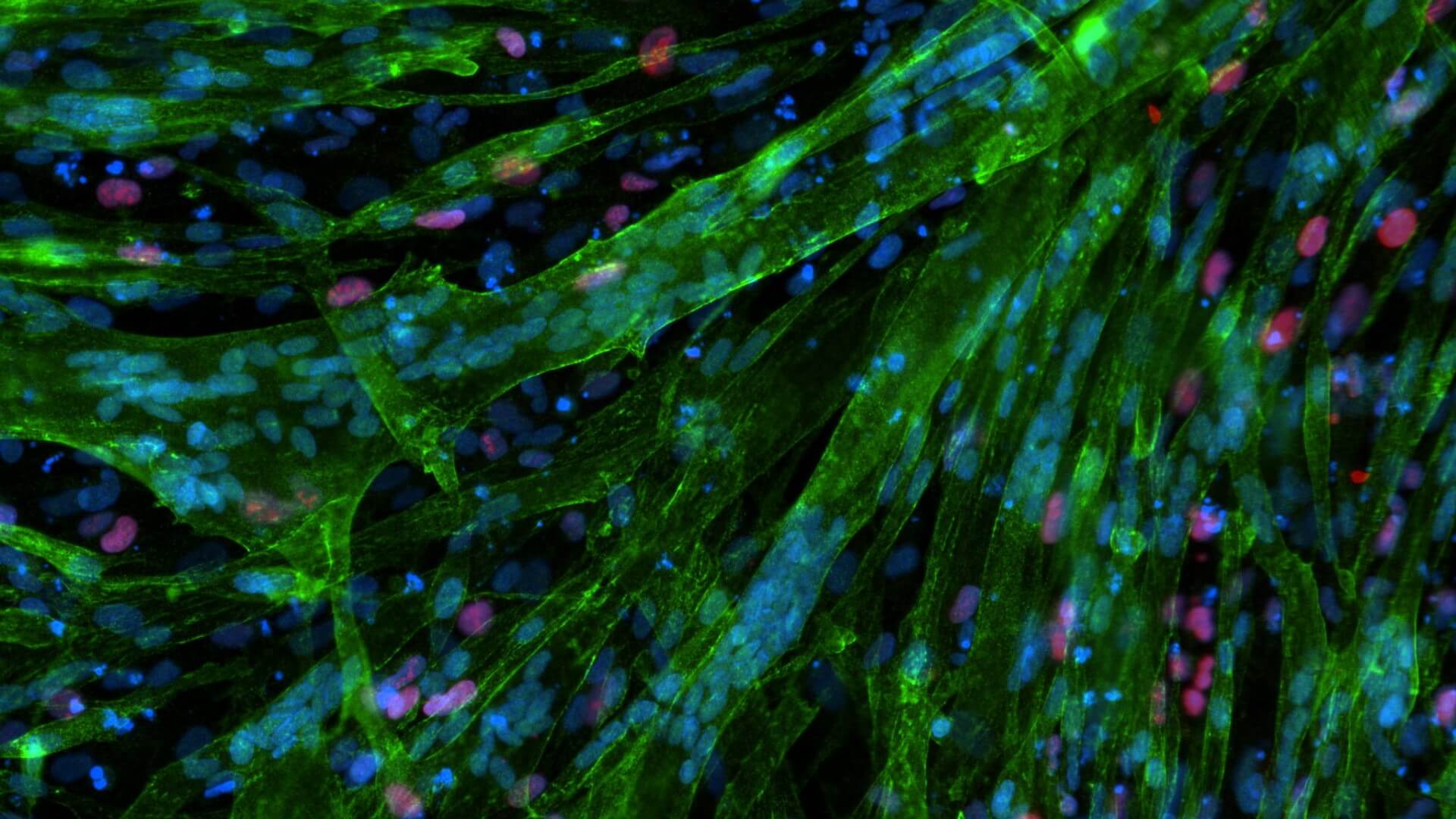
Myocea's GBC0905: Efficacy Recaptured in FSHD Primary Patient Biopsy Myotubes in a Dose-Dependent Fashion
Normal Myogenesis is Not Affected
GBC0905: Myocea’s Clinical Candidate to Treat FSHD
Orphan Drug Designation Granted, IND Enabling Studies Ongoing
- Confirmed DUX4 inhibitory activity in affected primary human cells
- EC50 < 100 nM across multiple affected human myoblast lines
- potential to treat both FSHD1/2, diverse patients
- no cellular toxicity, no myogenic effect(s), no off-target effects
- multiple back up series: same, different molecular pathways to reduce DUX4 tox
- Efficacy: dose-response across multiple hFSHD ex vivo/in vivo assays
- proximal DUX4 molecular targets identified, synergistic inhibition
- displays protective effect in DUX4-induced apoptosis assays @ < 200 nM
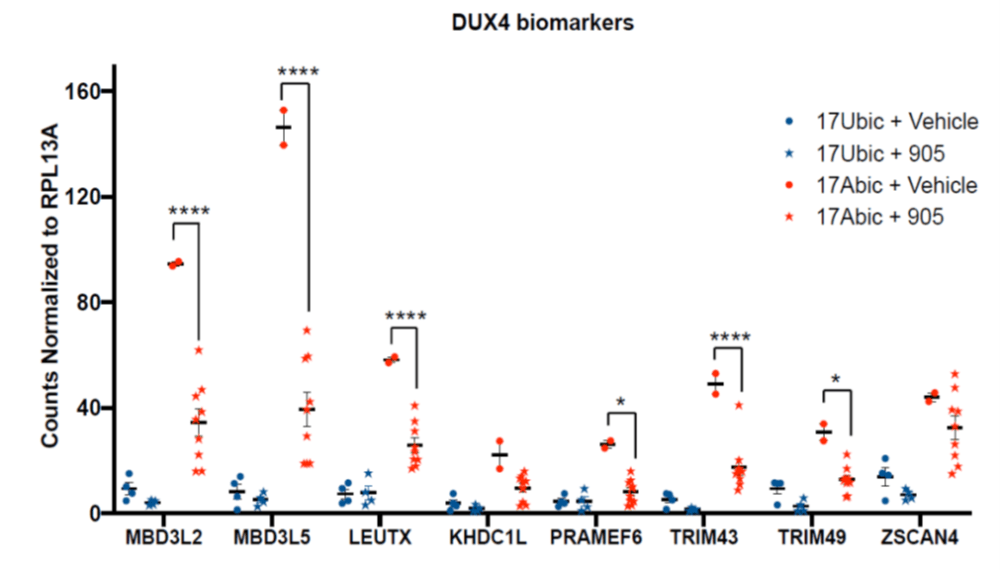
- dose-response effect on all key DUX4 target genes ex vivo and in vivo
- efficacy in FSHD xenograft model @ 10 mg/kg single dose
- IND for FSHD: ONGOING, ON TRACK
- safe, once daily oral agent with dosing at < 50 mg
- IND enabling studies ongoing
- clinical plan designed
GBC0905: Myocea's Clinical Candidate
Key Features
- Mode of action, GBC0905:
- Affects both DUX4 gene and respective toxic protein
- Blocks direct DUX4 gene binder/activator
- Inhibits multiple direct mechanisms of DUX4 activation
- Enhanced Therapeutic Window:
- Tested in >1,000 patients, 50 to 1,200 mg/day oral doses
- Regimen, GBC0905:
- Suitable for once daily or less frequent administration
- Compatible with synergistic therapies
- Diverse, proximal clinical markers of efficacy, disease progression:
- Relevant to GBC0905 target pharmacology
- mRNAs and proteins: muscle and plasma measurements
Contact Us
Want to say hello? Want to know more about us? Give us a call or drop us an email and we will get back to you as soon as we can.