Myocea:
We are Muscle-Strong
Learn more
Our Mission
From cells to therapeutics, organs and sustainable source of food
We convert cells from humans and animals into a competent, functional skeletal muscle tissue to model diseases, develop diverse therapeutic modalities, regenerate aged muscle, grow organs and make synthetic meat.
What we Do
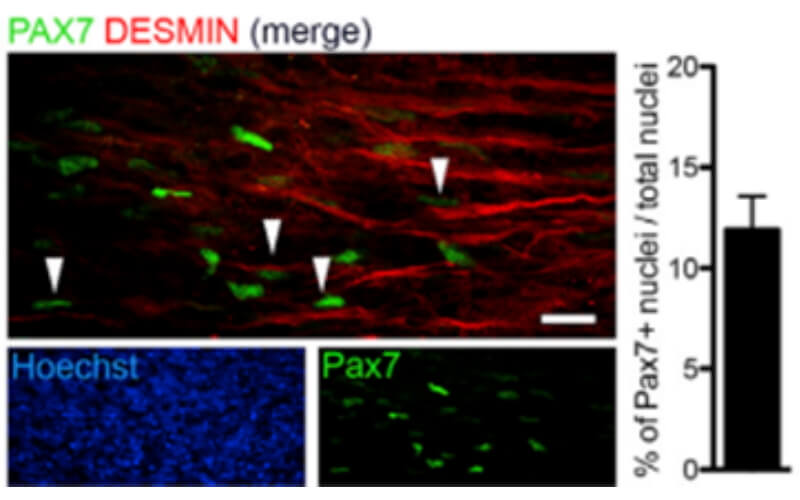
We use healthy or affected skeletal muscle progenitors cells to model neuromuscular diseases and to discover diverse therapeutics.
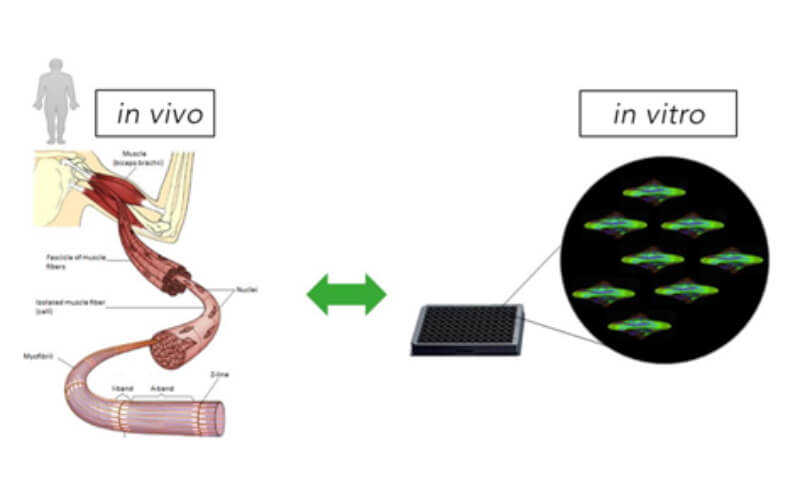
Our proprietary skeletal muscle differentiation platform reliably and reproducibly converts pluripotent or embryonic stem cells into satellite cells and myoblasts with high regenerative potential. This process does not require genetic intervention or cell sorting. It yields a homogeneous and physiologically competent population of cells and/or cultures immediately suitable for screening and advanced drug discovery.
High Yield
No Sorting
No Genetic Manipulation
Our Competitive Advantages
- Therapeutic candidate GBC0905 for the targeted treatment of FSHD
- Orphan Drug Designation (ODD) status granted by the FDA In May 2018;
- aimed to treat, delay and/or slow down progression of the disease;
- potently and synergistically targets multiple mechanisms of DUX4 toxicity;
- affects both DUX4 gene and respective protein activity;
- displays large efficacy-safety (therapeutic) window, suitable for once-daily oral administration;
- Skeletal Muscle Platform
- yields pure, fully competent progenitors with therapeutic regenerative potential including muscle stem cells (satellite cells)
- suitable for disease modeling, sophisticated screening, functional assays
- produces 2D/3D models of the skeletal muscle
- works with embryonic and pluripotent stem cells of diverse origin and genetic background
Pipeline
Accelerated discovery-to-clinic timelines for clinical development of diverse therapies to treat skeletal muscle.
Facioscapulohumeral Dystrophy (FSHD, TYPES I/II): GBC0905
Satellite Cells for Muscle Regeneration
Spinal Muscular Atrophy (Skeletal Muscle)
Kennedy’s Disease (SBMA)
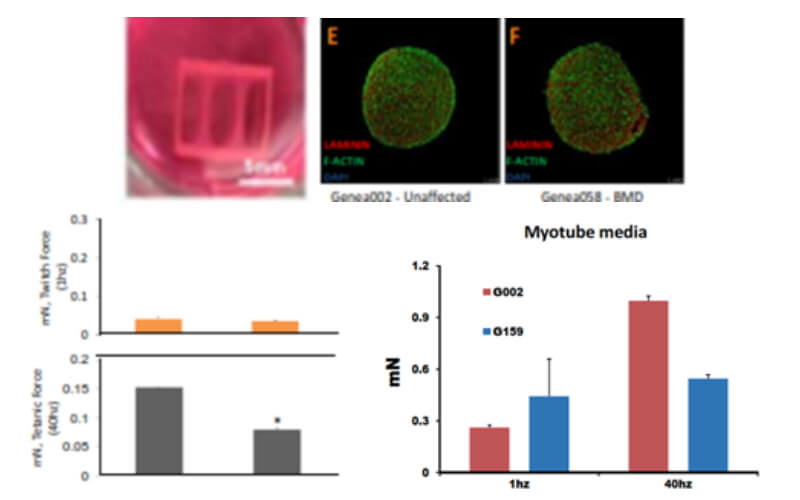
Generation of Improved 2D & 3D Skeletal Muscle Cultures
Mini-muscles: collaborative efforts
- Substrate firmness: align myofibers, promote maturation
- Stem cell derived organoids/3D cultures: realistic tissue milieu
- Clinically relevant assays
- contraction force, satellite cell niche, environment
- Myocea collaborates with:
- Industry: Vala Therapeutics, Cytoo on organoids, mini-muscle
- Academia: University of Massachusetts Medical School (Dr. Charles Emerson, Jr), Duke University (Dr. Nenad Bursac)
- Advocacy: FSH Society, FSH-MD Support Group UK
- Media: AMSBio, Aurorea, Funakoshi
Timeline
-
Genea Biocells Founded in Australia 2012Genea Biocells, the predecessor of Myocea, was founded by Genea, a leading IVF company from Australia with the world’s largest embryonic stem cell bank, to focus on disease modeling using human embryonic stem cells
-
Genea Biocells moves to the U.S. 2014Genea Biocells established business in San Diego, U.S. to focus on developing therapeutic pipeline to treat muscular dystrophies including facioscapulohumeral dystrophy (“FSHD”)
-
Therapeutic candidate GBC0905 to treat FSHD discovered 2018Genea Biocells received an Orphan Drug Designation from FDA for its small molecule GBC0905 as a lead agent to treat FSHD
-
Operations restarted as Myocea, Inc. May-June 2019Subsequent to the sale of Genea in late 2018, operations restarted as Myocea, Inc. with all key intellectual property and know-how retained
-
IND enabling studies for GBC0905 2020Operations re-started in San Diego, CA; IND enabling studies and clinical evaluation of GBC0905, skeletal muscle media and satellite-like cells regenerative medicine
-
IND filing and anticipated Phases I/II clinical studies 2021Projected IND filing and start of Phase I clinical studies for GBC0905
- Management Team
- Board of Directors
- Scientific Advisory Board
Management Team

Charles Martin
18 years experience in cell/stem cell biology focused on laboratory applications, scale up, assay development, production and automation; experienced in clinical regulatory and biosafety disciplines; Director of Operations.

Louis Villalba
25 years of industry experience in developing and commercializing innovative therapies; Business Development, currently the CEO of Genea Biomedx, Non-Executive Director to support Myocea’s fundraising activities.
Board of Directors

Alex Kiselyov, PhD
PhD, Founder and Chairman of the Board
Over two decades of drug discovery and development expertise in pharma and biotech companies including Amgen, ImClone/Lilly, deCODE, Genea Biocells; diverse modalities in mid/late stages of clinical developments to treat oncology, CNS, metabolic and neuromuscular disorders with specific emphasis on rare diseases; over 170 peer-reviewed publications and over 60 granted patents and patent applications.

Joseph Tam, PhD
PhD; over 45 years of experience in molecular diagnostics, founder and board of director of Pangenia Inc.

Louis Villalba
25 years of industry experience in developing and commercializing innovative therapies; currently the CEO of Genea Biomedx
Scientific Advisory Board

Charlie Emerson, Jr., PhD
Dr. Emerson is a key opinion leader in the field of muscular disorders. His research has focused on defining transcriptional networks and signaling pathways that control the specification and differentiation of skeletal muscle progenitors. Current studies utilize iPSC and xenograft technologies to model the molecular pathology of facioscapulohumeral (FSHD) and LGMD2i muscular dystrophies.

Lawrence Hayward, PhD
Dr. Lawrence Hayward is a physician-scientist providing care since 2000 for patients in the UMMS MDA Neuromuscular Clinic. Dr. Hayward serves as Co-Director of the multidisciplinary FSH Muscular Dystrophy Clinic. His research group focuses on defining molecular mechanisms that cause neuromuscular diseases including ALS, FSHD, and hyperkalemic periodic paralysis in order to design effective therapeutics for these conditions.

Kit Lam, MD, PhD
Dr. Lam is board certified in both Internal Medicine and Medical Oncology. He was on the faculty of the University of Arizona until June 1999, when he joined UC Davis School of Medicine as the Division Chief of Hematology/Oncology, Beginning April 1, 2010, he became the Chair of Department of Biochemistry and Molecular Medicine. He is both a practicing medical oncologist and a laboratory investigator.
Contact Us
Want to say hello? Want to know more about us? Give us a call or drop us an email and we will get back to you as soon as we can.